Stability Test of Medical Facilities
Verification services to stability test in medical facilities operating theater
Safety verification in medical facilities has a direct impact on the health of patients, outpatients, and medical staff.
KFTL, as a accredited testing laboratory by KOLAS, conducts verification and performance evaluation of HEPA filters, filtration performance of air purifiers, differential pressure evaluation of isolation facilities, and air cleanliness evaluation of operating theater in medical facilities

When tested with fine aerosol (DOP) particles, the removal efficiency shall be maintained at least 99.97%.
ASME N510 , ASME AG-1 , KEPIC MH , KS M ISO 14644-3
+ When the first installation or when static pressure management standards for filtration facilities are exceeded
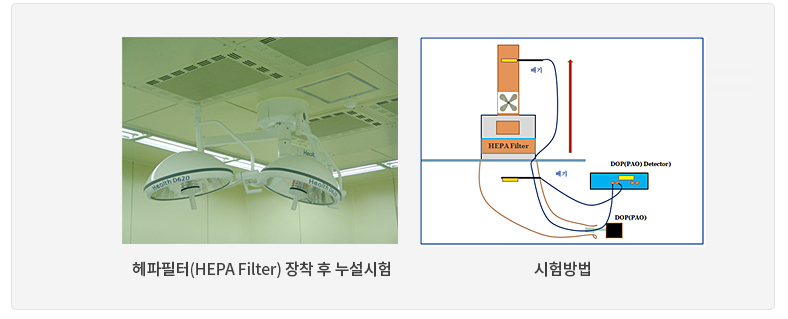
When tested with fine aerosol particles, the mechanical removal efficiency at the rated flow rate shall be maintained at least 99.95%.
ASME N510, KS I ISO 14644-3
+ Industrial Air Purification Facility (24 months)
+ Cleanroom/Clean bench: ISO 1 to 9 grades (24 months)
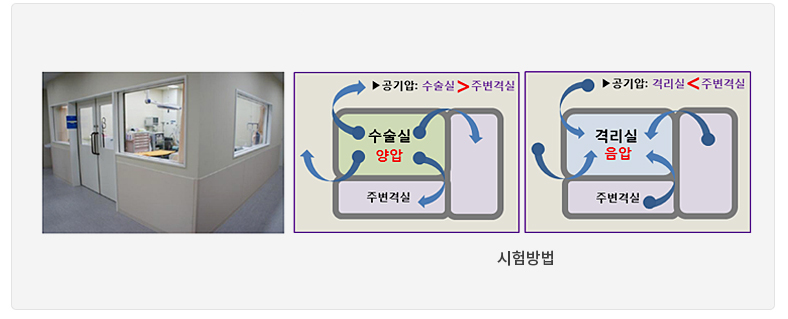
Isolation Room: Keep the pressure lower than the surrounding area [To prevent infection from spreading outside from the isolation room]
KS I ISO 14644-3
+ Cleanroom/Clean bench: ISO 1 to 9 grades (12 months)





